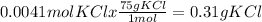Answer:
0.31g KCl
Step-by-step explanation:
Before we can start, we have to wonder, how many moles of KCl is formed from KClO3? To figure that out we have to make a balanced equation.
KClO3 -> KCl + O2
How do I know it makes O2? Looking back at the problem they're asking for KCl, which has less ions than KClO3 so it must have been broken down. So we can say it's a decomposition type of reaction. We can't just slap on O3, we know oxygen is one of our diatomic elements that usually exists as O2 so we'll put it down as O2 and balance our equation.
2KClO3 -> 2KCl + 3O2
Now that we know every 2 moles of KClO3 makes 2 moles of KCl, this is our mole to mole ratio. Knowing the ratio between the 2 compounds in our problem is the stepping stone from converting from grams KClO3 -> moles KClO3 -> moles KCl -> grams KCl.
1. Let's first convert grams KClO3 -> moles KClO3
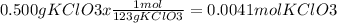
2. Convert moles KClO3 -> moles of KCl using our mole to mole ratio
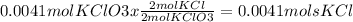
3. Convert moles KCl -> grams KCl