Answer:
11.6 mol O₂
Step-by-step explanation:
- C₇H₁₆ + 11 O₂ → 7 CO₂ + 8 H₂O
In order to solve this problem we need to convert moles of carbon dioxide (CO₂) into moles of oxygen gas (O₂). To do so we'll use a conversion factor containing the stoichiometric coefficients of the balanced reaction:
- 7.4 mol CO₂ *
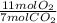 = 11.6 mol O₂
= 11.6 mol O₂