Answer:
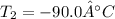
Step-by-step explanation:
Hello!
In this case, according to the given description of how the temperature changes for aluminum in agreement to the loss of heat of 6120.0 J, we can use the following equation:
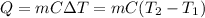
Thus, by knowing Q, m, C and the initial temperature, we are able to obtain:
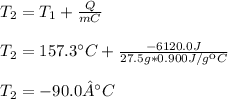
Regards!