Answer:
The pressure of the gas is 30.3072 atm.
Step-by-step explanation:
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P * V = n * R * T
In this case:
- P= ?
- V= 2.5 L
- n= 3 moles
- R= 0.082
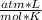
- T= 35 C= 308 K (being 0 C= 273 K)
Replacing:
P* 2.5 L= 3 moles* 0.082
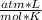 *308 K
*308 K
Solving:
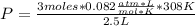
P= 30.3072 atm
The pressure of the gas is 30.3072 atm.