Answer:

Step-by-step explanation:
Molarity is the concentration measurement equal to moles per liter.
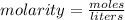
We have 26.0 grams of HCl and 1.00 liters of water. We must convert grams of HCl to moles.
To convert from grams to moles, the molar mass is used. Usually we find these values on the Periodic Table, but they are already provided. The molar mass of hydrochloric acid is 36.0 grams per mole. Use this number as a ratio.
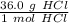
Multiply by the given number of grams: 26.0
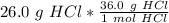
Flip the ratio so the grams of hydrochloric acid cancel.
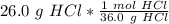
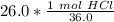
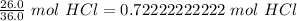
Now we know the moles and liters.
- 0.72222222222 mol HCl
- 1.00 L
Substitute these values into the molarity formula.
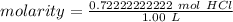
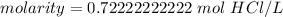
The original measurements of grams and liters have 3 significant figures, so our answer must have the same. For the number we calculated, that is the thousandth place.
The 2 tells us to leave the 2 in the thousandth place.
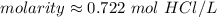
1 mole per liter is equal to 1 molar, or M. Change the units.
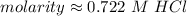
The molarity of this solution is approximately 0.722 M HCl