Answer: The molarity of this solution is 2.5 M.
Step-by-step explanation:
Given :
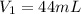 ,
,
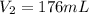
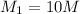
Therefore, the value of molarity of the solution is calculated using following formula.
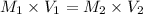
Substitute the values into above formula as follows.
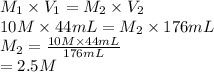
Hence, molarity of the solution is 2.5 M.