Answer:
Ionization Energy = 9 eV
Step-by-step explanation:
If we apply the law of conservation of energy to the given situation, we will get the following equation:
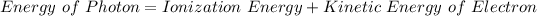
where,
Energy of Photon = 10 eV
Ionization Energy = ?
Kinetic Energy of Electrons = 1 eV
Therefore,
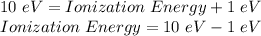
Ionization Energy = 9 eV