Answer:
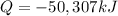
Step-by-step explanation:
Hello there!
In this case, since following reaction:
C2H4+3 O2-> 2CO2 + 2H2O
Has an enthalpy of combustion of ethene is about -1411.1 kJ/mol (can be found on goo gle), it is possible to calculate the total amount of energy that is released when 1000 grams of ethene are burned as shown below:
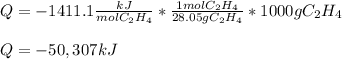
Best regards!