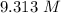Answer:
The correct response is "9.313 M".
Step-by-step explanation:
According to the question, the values are:
Moles of solute,
= 0.57 moles
Liters of solution,
= 61.2 mL
or,
= 0.0612 L
Now,
The molarity will be:
=
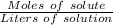
On putting the given the values in the above formula, we get
=
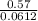
=