Answer: The balanced equation for the given reaction is
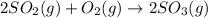 .
.
Step-by-step explanation:
A chemical equation which contains same number of atoms on both reactant and product side.
For example,
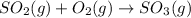
Here, number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
To balance this equation, multiply
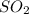 by 2 on reactant side and multiply
by 2 on reactant side and multiply
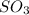 by 2. Hence, the equation will be re-written as follows.
by 2. Hence, the equation will be re-written as follows.
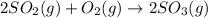
Here, number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
Now, there are same number of atoms on both reactant and product side. So, this equation is balanced.
Thus, we can conclude that the balanced equation for the given reaction is
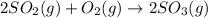 .
.