Answer:
a) p = 1.381 10⁻²⁹ kg m / s, b) λ = 4.80 10⁻⁵ m,
c) λ/a₀ = 9.6 10⁴ = 10⁵, λ/aₙ = 4.80 10⁹
Step-by-step explanation:
a) as the neutral goes at speeds much lower than the speed of light we can use the classical relationships, as the neutral all the thermal energy is the energy of the neutron
E = p c
K T = pc
p = k T / c
p =
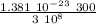
p = 1.381 10⁻²⁹ kg m / s
b) the expression for the de Broglie wavelength
p = h / λ
λ = h / p
λ =
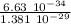
λ = 4.80 10⁻⁵ m
c) let's compare this size with the size of atoms a₀ = 0.5 10⁻⁹ m
λ/a₀ =
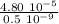
λ/a₀ = 9.6 10⁴ = 10⁵
c) let's compare with the atomic nucleus size aₙ = 10⁻¹⁴ m
λ/aₙ =
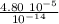
λ/aₙ = 4.80 10⁹