Answer:
The mass of the solution is 120 g.
Step-by-step explanation:
The mass of the solution is given by:
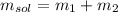
Where:
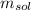 : is the mass of the solution
: is the mass of the solution
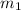 : is the mass of the solvent
: is the mass of the solvent
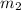 : is the mass of the solute
: is the mass of the solute
In the solution, the solvent is the majority compound (in mass) and the solute is the minority (in mass), so the solvent is the water and the solute is sodium chloride.
Hence, the mass of the solution is:
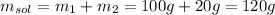
I hope it helps you!