Answer:
Pressure increases to 1.16 atm if the temperature changes to 317 K.
Step-by-step explanation:
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C (or 273 K) are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
On the other hand, Gay Lussac's law indicates that when there is a constant volume, as the temperature increases, the pressure of the gas increases. And when the temperature is decreased, the pressure of the gas decreases. This law mathematically indicates that the quotient between pressure and temperature is constant:
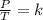
Studying an initial state 1 and a final state 2 is fulfilled:
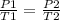
In this case:
- P1= 1 atm
- T1= 0 C= 273 K
- P2= ?
- T2= 317 K
Replacing:
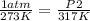
Solving:
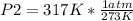
P2= 1.16 atm
Pressure increases to 1.16 atm if the temperature changes to 317 K.