Answer:
0.4 M
Step-by-step explanation:
The process that takes place in an aqueous K₂HPO₄ solution is:
First we calculate how many K₂HPO₄ moles are there in 200 mL of a 0.2 M solution:
- 200 mL * 0.2 M = 40 mmol K₂HPO₄
Then we convert K₂HPO₄ moles into K⁺ moles, using the stoichiometric coefficients of the reaction above:
- 40 mmol K₂HPO₄ *
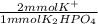 = 80 mmol K⁺
= 80 mmol K⁺
Finally we divide the number of K⁺ moles by the volume, to calculate the molarity:
- 80 mmol K⁺ / 200 mL = 0.4 M