Answer:
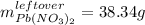
Step-by-step explanation:
Hello there!
In this case, given the chemical reaction by which the sodium chloride reacts with lead (II) nitrate and the former is the limiting reactant, it is possible to calculate the mass of lead (II) nitrate that are actually consumed according to the 2:1 mole ratio between them:
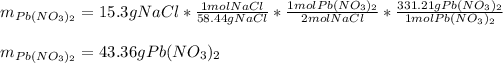
Thus, the leftover of lead (II) nitrate is:
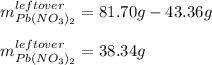
Best regards!