Answer:
See explanation.
Step-by-step explanation:
Hello there!
In this case, for the required chemical reactions, we proceed as follows, by considering that the left-handed elements stand for the cations and the right-handed ones for the anions:
1:
1.
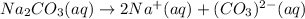
2.
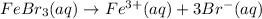
3.
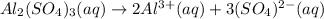
2: In this case, we can represent the formula equation first according to the neutralization reaction:
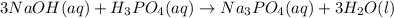
Which is worded as: three molecules of aqueous sodium hydroxide reacts with one molecule of aqueous phosphoric acid to yield one molecule of aqueous sodium chloride and three molecules of liquid water.
Regards!