Answer:
17.95 g
Step-by-step explanation:
The electrolysis of CaBr₂ → Ca²⁺ + 2Br⁻
Ca₂⁺ + 2e⁻ → Ca
Recall that:
Charge (Q) = current (I) × time (t)
Q = 10.0 × 2.4 × 3600
Q = 86400 C
Number of moles of electron =
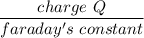

No of moles of Ca =
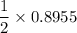
= 0.44775 moles
Mass of Ca = no of moles × molar mass of Ca
= 0.44775 mol × 40.08 g/mol
= 17.95 g