Answer: Molarity of the solution is 0.028 M.
Step-by-step explanation:
Given : Moles of sucrose (solute) = 0.112 mol
Volume of solution = 4.0 L
Molarity is the number of moles of solute dissolved in a liter of solvent.
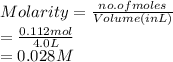
Thus, we can conclude that molarity of the solution is 0.028 M.