Answer: The compound formed between nitrogen (N) and nitrogen (N) a polar covalent bond because there is less than a 0.4 difference in electronegativity between the atoms.
Step-by-step explanation:
If electronegativity difference between two atoms is less than 0.4 then bond formed is a pure covalent bond.
If electronegativity difference between two atoms is between 0.4 to 1.8 then bond formed is a polar covalent bond.
If electronegativity difference between two atoms is greater than 1.8 then bond formed is an ionic bond.
The electronegativity value of a nitrogen atom is 3.04. Hence, the electronegativity difference of
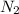 molecule is as follows.
molecule is as follows.
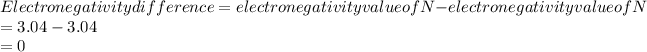 As the electronegativity difference is 0 which is less than 0.4.
As the electronegativity difference is 0 which is less than 0.4.
Hence, we can conclude that the compound formed between nitrogen (N) and nitrogen (N) a polar covalent bond because there is less than a 0.4 difference in electronegativity between the atoms.