Answer:
q = 0.384 C
Step-by-step explanation:
The total charge present at the head can be easily found out by multiplying the charge on a single electron with the total number of electrons present on the head:
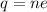
where,
q = total charge on head = ?
n = total no. of electrons on the head = 2.4 x 10¹⁸
e = charge on 1 electron = 1.6 x 10⁻¹⁹ C
Therefore,
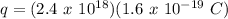
q = 0.384 C