Answer:
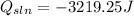
Step-by-step explanation:
Hello there!
In this case, for this calorimetry problem, it is possible for us to infer that the heat of the reaction of dissolution of KNO3 is absorbed by the solution composed by the former and water so that we can write:
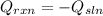
Thus, given the mass, specific heat and temperature of the solution, we plug in the data to obtain the heat absorbed, by the reaction:
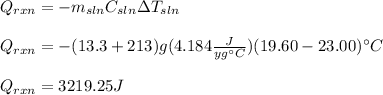
Also, we can say the the heat released by the solution was -3219.25 J.
Best regards!