Answer:
18 moles of O2 can be produced by letting 12.00 moles of KClO3 react
Step-by-step explanation:
The balanced equation is
2KCIO3 ---> 2 KCl + 3 O2
As per the reaction,
2 mole of KClO3 produces 3 mole of O2 gas
Hence, 12 moles of KClO3 will produce
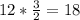
Hence, 18 moles of O2 can be produced by letting 12.00 moles of KClO3 react