Answer:
Time = 6 years
Step-by-step explanation:
First, we will calculate the no. of half life periods required to reduce the mass of Protactinium to the given value:
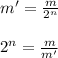
where,
n = no. of half-life periods = ?
m = initial mass = 86.3 g
m' = remaining mass = 10.8 g
Therefore,
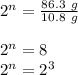
Since the bases are the same. Therefore equating powers:
n = 3
Now we calculate the time:
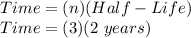
Time = 6 years