Answer:
10 molecules of NH₃.
Step-by-step explanation:
N₂ + 3H₂ --> 2NH₃
As the N₂ supply is unlimited, what we need to do to solve this problem is convert molecules of H₂ into molecules of NH₃. To do so we use the stoichiometric coefficients of the balanced reaction:
- 15 molecules H₂ *
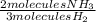 = 10 molecules NH₃
= 10 molecules NH₃
10 NH₃ molecules could be prepared from 15 molecules of H₂ and unlimited N₂.