Answer:
Q1: c. 7.2 g.
Q2: a. 0.42 M.
Step-by-step explanation:
Hello there!
In this case, according to the definition of molarity as moles of solute divided by volume of solution in liters, we can proceed as follows:
Q1: Here, given the molarity and volume we can calculate the moles of the sugar as follows:
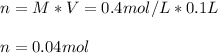
Next, since its molar mass is about 180 g/mol, the mass turns out:
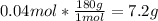
Therefore, the answer is c. 7.2 g.
Q2: Here, recalling the definition of molarity, we can just plug in the 0.629 moles and 1.500 L to obtain:
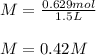
Therefore, the answer is a. 0.42 M.
Best regards!