Answer:
58.6 g.
Step-by-step explanation:
Hello there!
In this case, since the molecular formula of lithium thiocyanate is LiCNS and therefore its molar mass is 65.1 g/mol, it possible to perform the calculation of the mass of 0.900 moles of this substance by recalling the following equivalence statement:
1 mol = 65.1 g.
Thereby, we can calculate the required mass as shown below:
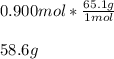
Best regards!