Answer:
The amount of moles of NaOH is 0.0495
Step-by-step explanation:
Molarity is a measure of concentration that is defined as the number of moles of solute that are dissolved in a given volume.
In symbolic form molarity is presented as:
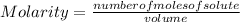
Molarity is expressed in units
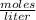 .
.
In this case:
- Molarity= 0.33 M
- Number of moles of solute= ?
- Volume= 0.75 L
Replacing in the definition of molarity:
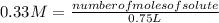
number of moles of solute= 0.33 M* 0.75 L
number of moles of solute= 0.0495 moles
The amount of moles of NaOH is 0.0495