Answer:
You must add 48.97 mL of water to make the 0.200 M diluted solution.
Step-by-step explanation:
In chemistry, dilution is the reduction in concentration of a chemical in a solution. In other words, it is the process of reducing the concentration of solute in solution, simply adding more solvent to the solution.
In a dilution, the quantity or mass of the solute is not changed but only that of the solvent. As only solvent is being added, by not increasing the amount of solute the concentration of the solute decreases.
The expression for the dilution calculations is:
Cinitial* Vinitial = Cfinal* Vfinal
In this case:
- Cinitial= 12 M
- Vinitial= 0.830 mL
- Cfinal= 0.200 M
- Vfinal= ?
Replacing:
12 M*0.830 mL= 0.200 M*Vfinal
Solving:
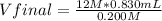
Vfinal= 49.8 mL
Since 0.830 mL is the volume you initially have of HCl, the amount of water you must add is:
49.8 mL - 0.830 mL= 48.97 mL
You must add 48.97 mL of water to make the 0.200 M diluted solution.