Answer:
3.97 psi
Step-by-step explanation:
The new pressure can be found by using the formula for Boyle's law which is
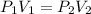
Since we're finding the new pressure
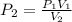
We have
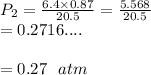
But
1 atm = 14.7 psi
Then 0.27 atm =
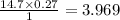
We have the final answer as
3.97 psi
Hope this helps you