Solution :
According to Chick's law
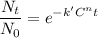
where, t = contact time
c = concentration of disinfectant
k' = lethality coefficient = 4.71
n = dilution coefficient = 1
4 log removal = % removal = 99.99
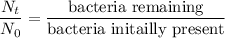
= 1 - R
= 1 - 0.9999
Now for plug flow reactor contact time,
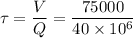
= 0.01875 days
= 27 minutes
For CSTR,
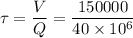
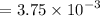 days
days
= 5.4 minute
There are 3 reactors, hence total contact time = 3 x 5.4
= 16.2 minute
Or
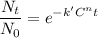
or
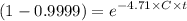
∴ C x t = 1.955
For PFR,
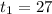 min
min
∴ C
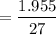 = 0.072 mg/L
= 0.072 mg/L
For CSIR,
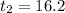 min
min
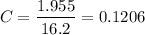 mg/L
mg/L
∴ Chlorine required for PFR in kg/day
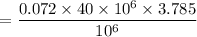 (1 gallon = 3.785 L)
(1 gallon = 3.785 L)
= 18.25 kg/day
Therefore we should go for PFR system.