Answer:
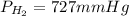
Step-by-step explanation:
Hello there!
In this case, according to the given data, it is possible to infer that the gas mixture lies on the 15.0 cm-high column of water, so that the total pressure or atmospheric pressure is given by:
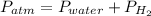
Thus, since the atmospheric pressure is 745 mmHg and the vapor pressure of water is 18 mmHg, the pressure of hydrogen turns out to be:
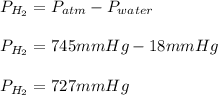
Best regards!