Answer:
the order of vibrational frequency is:
C ≡ N > C=N > C=S > S-C
Step-by-step explanation:
The bond's vibration frequency is determined using the following equation:
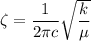
where;
reduced mass
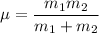
velcoity of light = c
force constant = k
The frequency of vibration, on the other hand, is inversely proportional to the atom's mass, because the heavier the atom, the lower the frequency.
In addition, the value of a bond's stretching frequency rises as the bond's intensity rises. As a result, the frequency is as follows:
triple > double > single
The reduced mass
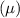 of C-N bond.
of C-N bond.
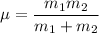
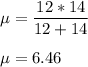
The reduced mass of C-S bond;
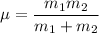
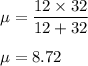
Thus, the order of vibrational frequency is:
C ≡ N > C=N > C=S > S-C