Answer:
0.172 M
Step-by-step explanation:
The reaction for the first titration is:
First we calculate how many HCl moles reacted, using the given concentration and volume:
- 19.6 mL * 0.189 M = 3.704 mmol HCl
As one HCl mol reacts with one NaOH mol, there are 3.704 NaOH mmoles in 25.0 mL of solution. With that in mind we determine the NaOH solution concentration:
- 3.704 mmol / 25.0 mL = 0.148 M
As for the second titration:
- H₃PO₄ + 3NaOH → Na₃PO₄ + 3H₂O
We determine how many NaOH moles reacted:
- 34.9 mL * 0.148 M = 5.165 mmol NaOH
Then we convert NaOH moles into H₃PO₄ moles, using the stoichiometric coefficients:
- 5.165 mmol NaOH *
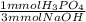 = 1.722 mmol H₃PO₄
= 1.722 mmol H₃PO₄
Finally we determine the H₃PO₄ solution concentration:
- 1.722 mmol / 10.0 mL = 0.172 M