Answer:
See explanation
Step-by-step explanation:
Hello there!
In this case, since the the concentrations are not given, and not even the Ksp, we can solve this problem by setting up the chemical equation, the equilibrium constant expression and the ICE table only:
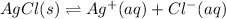
Next, the equilibrium expression according to the produced aqueous species as the solid silver chloride is not involved in there:
![Ksp=[Ag^+][Cl^-]](https://img.qammunity.org/2022/formulas/chemistry/college/258g5rj7wu5xs4rwcazox93rxl9nqg1bpv.png)
And therefore, the ICE table, in which x stands for the molar solubility of the silver chloride:
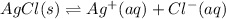
I - 0 0
C - +x +x
E - x x
Which leads to the following modified equilibrium expression:
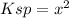
Unfortunately, values were not given, and they cannot be arbitrarily assigned or assumed.
Regards!