Answer:
The density of the 1,000,000 kg iceberg = The density of the 10 g iceberg
Step-by-step explanation:
The given quantities of iceberg that will be the basis of the comparison of the densities of the iceberg;
The mass of the iceberg = 1,000,000 kg
The mass of the ice cube taken from the iceberg = 10 g
The density of a substance, is the measure of the mass of the substance per volume of the substance, it is a constant for the substance
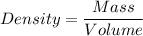
For the iceberg, the density of the iceberg is the property that indicates the volume occupied by a given mass of the iceberg
Because of density, we can estimate that the volume occupied by a 1,000 kg iceberg is 10 times the volume occupied by a 100 kg iceberg
We can write
The density of the 100 kg iceberg = 100 kg/(x m³)
The density of the 1,000 kg iceberg = 1,000 kg/(10 × x m³) = 100 kg/(x m³)
Therefore, the density of the 100 kg iceberg = The density of the 1,000 kg iceberg
Similarly,
The density of the 1,000,000 kg iceberg = The density of the 10 g iceberg because the 10 g iceberg was obtained from the 1,000,000 kg iceberg, and therefore, they have the same density.