Answer: A heat energy of 50160 joules are required to heat 15 grams of ice from
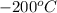 to
to
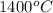 .
.
Step-by-step explanation:
Given : mass of ice = 15 g
Convert grams into kg as follows,
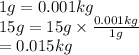
Initial temperature =
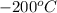
Final temperature =
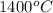
Formula used to calculate the amount of heat energy is as follows.
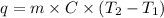
where,
q = heat energy
m = mass of substance
C = specific heat of substance (ice here has C =
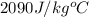 )
)
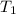 = initial temperature
= initial temperature
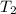 = final temperature
= final temperature
Substitute the values into above formula as follows.
![q = m * C * (T_(2) - T_(1))\\= 0.015 kg * 2090 J/kg^(o)C * [1400 - (-200)]^(o)C\\= 50160 J](https://img.qammunity.org/2022/formulas/chemistry/high-school/hl57uqlhunnh7hrbhz7hx5hgx55inadyp8.png)
Thus, we can conclude that 50160 joules are required to heat 15 grams of ice from
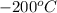 to
to
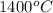 .
.