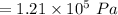Answer:
The correct answer is "
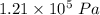 ".
".
Step-by-step explanation:
The given values are:
Number of moles,
n = 2.5 moles
Volume,
V = 45 liters
Temperature,
T = 261 K
As we know,
⇒

or,
⇒
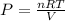
On substituting the values, we get
⇒
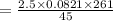
⇒
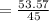
⇒
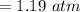
On converting it in Pa, we get
⇒
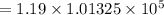
⇒