Answer: There are
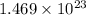 molecules present in 7.62 L of
molecules present in 7.62 L of
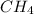 at
at
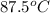 and 722 torr.
and 722 torr.
Step-by-step explanation:
Given : Volume = 7.62 L
Temperature =
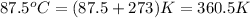
Pressure = 722 torr
1 torr = 0.00131579
Converting torr into atm as follows.
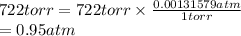
Therefore, using the ideal gas equation the number of moles are calculated as follows.
PV = nRT
where,
P = pressure
V = volume
n = number of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.

According to the mole concept, 1 mole of every substance contains
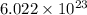 atoms. Hence, number of atoms or molecules present in 0.244 mol are calculated as follows.
atoms. Hence, number of atoms or molecules present in 0.244 mol are calculated as follows.
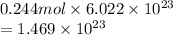
Thus, we can conclude that there are
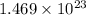 molecules present in 7.62 L of
molecules present in 7.62 L of
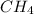 at
at
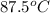 and 722 torr.
and 722 torr.