Answer:
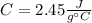
Step-by-step explanation:
Hello there!
In this case, since the thermodynamic definition of heat in terms of mass, specific heat and temperatures is given by:
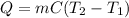
We are to calculate the specific heat of the ethanol as shown below:
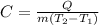
Thus, by plugging it the given data we can obtain:
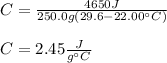
Regards!