Answer:
Y = 62.5%
Step-by-step explanation:
Hello there!
In this case, for the given chemical reaction whereby carbon dioxide is produced in excess oxygen, it is firstly necessary to calculate the theoretical yield of the former throughout the reacted 10 grams of carbon monoxide:
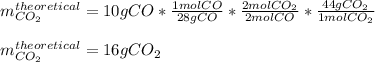
Finally, given the actual yield of the CO2-product, we can calculate the percent yield as shown below:
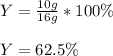
Best regards!