Answer: The concentration of hydrogen ions in the given solution is
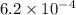 .
.
Step-by-step explanation:
Given : pH = 4.32
pH of a substance or solution is the negative logarithm of hydrogen ion concentration.
![pH = - log [H^(+)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/7qnomb01z9o3qpasap8cj8in33szh8kqym.png)
Substitute the value into above formula as follows.
![pH = - log [H^(+)]\\= - log (4.32)\\= 6.2 * 10^(-4)](https://img.qammunity.org/2022/formulas/chemistry/high-school/agm0652x6zietgttlv71284xy13sf3aa4r.png)
Thus, we can conclude that the concentration of hydrogen ions in the given solution is
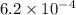 .
.