Answer:
49.2 g/mol
Step-by-step explanation:
Let's first take account of what we have and convert them into the correct units.
Volume= 236 mL x (
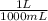 ) = .236 L
) = .236 L
Pressure= 740 mm Hg x (
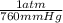 )= 0.97 atm
)= 0.97 atm
Temperature= 22C + 273= 295 K
mass= 0.443 g
Molar mass is in grams per mole, or MM=
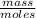 or MM=
or MM=
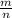 . They're all the same.
. They're all the same.
We have mass (0.443 g) we just need moles. We can find moles with the ideal gas constant PV=nRT. We want to solve for n, so we'll rearrange it to be
n=
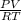 , where R (constant)= 0.082 L atm mol-1 K-1
, where R (constant)= 0.082 L atm mol-1 K-1
Let's plug in what we know.
n=
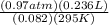
n= 0.009 mol
Let's look back at MM=
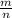 and plug in what we know.
and plug in what we know.
MM=
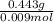
MM= 49.2 g/mol