Answer:
The correct option is option;
A. 1.58 mol
Step-by-step explanation:
The given balanced chemical reaction is presented as follows;
2Al₂O₃ → 4Al + 3O₂
The given mass of Al produced = 85.5 grams
The given molar mass of Al₂O₃ = 85.962 g/mol
The given molar mass of O₂ = 31.998 g/mol
The given molar mass of Al = 26.982 g/mol
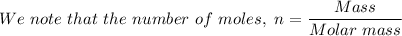
For the given produced Al, n = 85.5 g/(26.982 g/mol) ≈ 3.169 moles
From the given reaction, we have;
2 moles of Al₂O₃ produces 4 moles of Al
∴ 2/4 moles of Al₂O₃ produces 4/4 = 1 mole of Al
2/4 × 3.169 = 1.5845 moles of Al₂O₃ produces 1 × 3.169 = 3.169 moles of Al
∴ 1.5845 ≈ 1.58 moles of Al₂O₃ produces 3.169 moles of Al ≡ 85.5 grams of Al
The correct option is approximately 1.58 moles of Al₂O₃.