Answer:
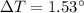
Step-by-step explanation:
Given that,
Mass of a lead bullet, m = 0.02 kg
Speed of the bullet, v = 200 m/s
It strikes with an armor plate 2kg at rest. All its kinetic energy is transferred to heat. We need to find the change in temperature.
A/c to the law of conservation of energy,
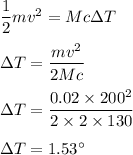
So, the required change in temperature is equal to
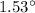 .
.