Answer:
9.47 mL
Step-by-step explanation:
The reaction that takes place is:
- 2KOH + H₂SO₄ → K₂SO₄ + 2H₂O
First we calculate how many KOH moles reacted, using the given concentration and volume of KOH solution:
- 0.061 mol/L = 0.061 mmol/mL
- 0.061 mmol/mL * 26.7 mL = 1.6287 mmol KOH
Then we convert KOH moles into H₂SO₄ moles, using the stoichiometric coefficients:
- 1.6287 mmol KOH *
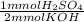 = 0.8144 mmol H₂SO₄
= 0.8144 mmol H₂SO₄
Finally we calculate the required volume of the H₂SO₄ solution, using the number of moles and given concentration:
- 0.8144 mmol ÷ 0.086 mmol/mL = 9.47 mL