Answer:
0.68 M
Step-by-step explanation:
The concentration of a solute refers to Molarity=
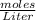
We need to find moles, we can do that by using stoichiometry to convert 6g > moles using molar mass.
Molar mass of C6H8O6= 176 g/mol; this is our key to converting the amount we have to moles.
6g C6H8O6 x
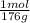 C6H8O6 = 0.034 mol C6H8O6
C6H8O6 = 0.034 mol C6H8O6
Now we need our volume per drop.
We know 1000 mL=1 L
We have 20 drops per 1 mL, so 1 mL / 20 = 0.05 mL per drop. But we need Liters, so we'll convert it to Liters.
0.05 mL x
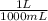 = 0.00005L
= 0.00005L
Now we can solve for Molarity.
Molarity of C6H8O6 =
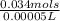 = 0.068M of C6H8O6
= 0.068M of C6H8O6