Answer:
If kept in Celsius: 0.15 L
If kept in Kelvin: 0.89 L = 0.90 L
Step-by-step explanation:
This formula can be remembered by just remembering the ideal gas law formula, PV=nRT
P= pressure, V= volume, n= moles, R=constant, and T= temperature.
Let's take away what is constant, particles/moles, pressure, and R.
so what we have is P=T ->
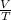 =x, we want to know how it changes though so we can say that
=x, we want to know how it changes though so we can say that
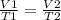 . Where P1 and T1 are for the air in your lungs that you expel, the P2 and T2 is the air now in your surroundings after you exhale.
. Where P1 and T1 are for the air in your lungs that you expel, the P2 and T2 is the air now in your surroundings after you exhale.
Let's plug it in and solve for V2.
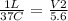
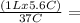 V2
V2
0.15 L = V2