Answer:
-635 J or -.635 kJ
Step-by-step explanation:
ΔHrxn= The change/difference in enthalpy between the products and reactants. Here the ΔHrxn tells us there is -902 kJ released per mole. So we can say we 4 mols of NH3 release -902 kJ. This can also be applied to the other compounds, where 5 mols of O2 release -902 kJ.
Keeping that in mind, we are looking for the heat released for 47.9g of NH3, let's convert grams > mols > kJ using the connection we made above.
1. We first need the molar mass of NH3 (17g) to convert to moles.
(47.9 g NH3) x
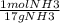 x
x
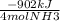 = -635 J
= -635 J
2. If we need it in kJ, we can convert knowing that...
1000 J= 1 kJ
-635 J x
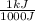 = -.635 kJ
= -.635 kJ