Answer:
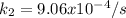
Step-by-step explanation:
Hello there!
In this case, since the activation energy, rate law and temperature, when variable, are related to each other as shown below:
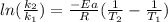
Thus, when solving for the rate constant at 682 K, we will obtain:
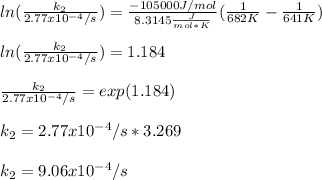
Best regards!