Answer:

Step-by-step explanation:
To convert from moles to mass, the molar mass is used. This is the grams per mole for a substance.
The values are found on the Periodic Table. They are the same as the atomic masses, but the units are grams per mole instead.
We are given this compound: C₆H₁₄. Look up the molar masses for the individual elements first: carbon and hydrogen.
- C: 12.011 g/mol
- H: 1.008 g/mol
Check the subscripts, which tell us the number of atoms per molecule. There are 6 carbon atoms and 14 hydrogen atoms. Multiply the molar mass by that number, then add them together.
- C₆: 12.011*6=72.066 g/mol
- H₁₄: 1.008*14=14.112 g/mol
- C₆H₁₄: 72.066+14.112=86.178 g/mol
Use the molar mass as a ratio.
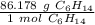
Multiply by 1.5 moles.
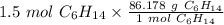
The moles of C₆H₁₄ will cancel each other out.
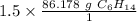
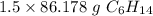
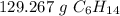
1.5 moles of C₆H₁₄ is equal to 129.267 grams of C₆H₁₄